
Introduction
In the article below, we will cover the importance of keeping up to date with regulatory standards. A reminder of the final transition date for the EMC collateral norm in the IEC 60601 medical device standard and a brief introduction to the most prominent changes.
FDA – Electromagnetic Compatibility (EMC)
A medical device is compatible with its electromagnetic environment when it does not emit levels of electromagnetic energy that causes electromagnetic interference in other nearby devices. A medical device can be vulnerable to electromagnetic interference if the levels of electromagnetic energy in its environment exceed the electromagnetic immunity (resistance) for which the device was designed and tested. The different forms of electromagnetic energy that can cause electromagnetic interference are conducted, radiated, and electrostatic discharge. Electromagnetic interference problems with medical devices can be very complex, not only from a technical point of view but also from the view of public health issues and solutions.
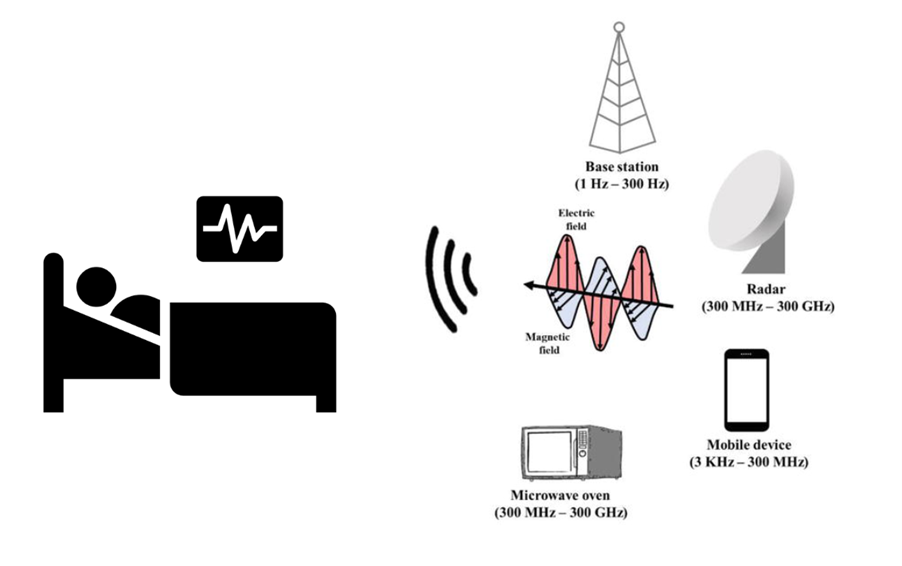
Updates on the regulatory standard
Nowadays, it is unusual for an electrical or electronic device to have no interaction with other devices or any kind of signal. Currently, there are more and more devices connected between them. In the era of the Internet of Things, all devices have some kind of communication, reception, or emission, due to this, the need arises to ensure the functioning without interfering with each other. This fact is even more decisive when we talk about the medical device industry, focused on improving the health of patients or even guaranteeing their lives.
Medical devices must ensure its proper functioning and avoid interferences from any source (radio, smartphones, etc.), not necessarily from a nearby device, that can disrupt them and cause vital malfunctions that can affect patient’s life. Similarly, medical devices should not emit signals with high electromagnetic levels that cause other devices in the environment to be adversely affected.
The IEC 60601-1-2 standard on electromagnetic disturbances requires levels of electromagnetic compatibility to ensure that different medical devices work together.
Transition period published by the FDA and Implications
The FDA will end the transition period as of 17 December 2023, after which date, declarations of conformity for the previous versions of the standards will be declined.
Implications
Changes in standards require new tests, updates in risk management, changes in documentation and other general changes. Some points that should be considered:
- Risk analysis is now a fundamental part of establishing levels of testing.
- Electromagnetic compatibility must be integrated with risk analysis to cover the safety and basic functionalities of the medical device.
- Depending on the environment of use of the medical device, specific immunity levels must be met (professional healthcare, home healthcare, special).
- New technologies must be considered, the number of communication networks has not stopped growing and there are many types of radio frequency transmitters (Wi-Fi, Bluetooth, RFID, LTE, 5G, Wireless Charging, etc.).
Considerations
It is complex to keep up to date due to the number of collateral and alternative standards that can be applied to a medical device depending on its functions and environment.
Updates in regulatory standards can sometimes come as a surprise to medical device manufacturers. A specific test plan is crucial to define essential performance and acceptance criteria, performance, description and intended use environment, category and testing, deviations, test configurations, connections, voltages, frequency list, software, and modes of operation, among others.
Make sure you develop and test your medical devices in compliance with the new requirements of the most current standards. To avoid any problems in the future, consider the dates when you will approve your medical devices and what standards will be in effect at that time.
If you want your company to be prepared for the upcoming changes regarding the standards applicable to medical equipment, contact our experts at D.med Software, they can help your team prepare or solve the upcoming changes and ensure that your project does not suffer inconvenience.
Additional notes
The date mentioned in the article does not refer exclusively to compliance with IEC 60601-1-2 4.1, other collateral standards have also been updated and should be considered:
IEC 60601-1 Edition 3.1 | -> | IEC 60601-1 Edition 3.2 |
IEC 60601-1-2 Edition 4.0 | -> | IEC 60601-1-2 Edition 4.1 |
IEC 60601-1-3 Edition 2.1 | -> | IEC 60601-1-3 Edition 2.2 |
IEC 60601-1-6 Edition 3.1 | -> | IEC 60601-1-6 Edition 3.2 |
IEC 60601-1-8 Edition 2.1 | -> | IEC 60601-1-8 Edition 2.2 |
IEC 60601-1-10 Edition 1.1 | -> | IEC 60601-1-10 Edition 1.2 |
IEC 60601-1-11 Edition 2.0 | -> | IEC 60601-1-11 Edition 2.1 |
IEC 60601-1-12 Edition 1.0 | -> | IEC 60601-1-12 Edition 1.1 |